Since its discovery in human blood plasma about 70 years ago, circulating cell-free DNA (cfDNA) has become an attractive subject of research as noninvasive disease biomarker. The interest in clinical applications has gained an exponential increase, making it a popular and potential target in a wide range of research areas.
cfDNA can be found in different body fluids, both in healthy and not healthy subjects. The recent and rapid development of new molecular techniques is promoting the study and the identification of cfDNA, holding the key to minimally invasive diagnostics, improving disease monitoring, clinical decision, and patients’ outcome.
cfDNA has already given a huge impact on prenatal medicine, and it could become, in the next future, the standard of care also in other fields, from oncology to transplant medicine and cardiovascular diseases.
Application of cfDNA:
Tumor Diagnosis (Liquid Biopsy):
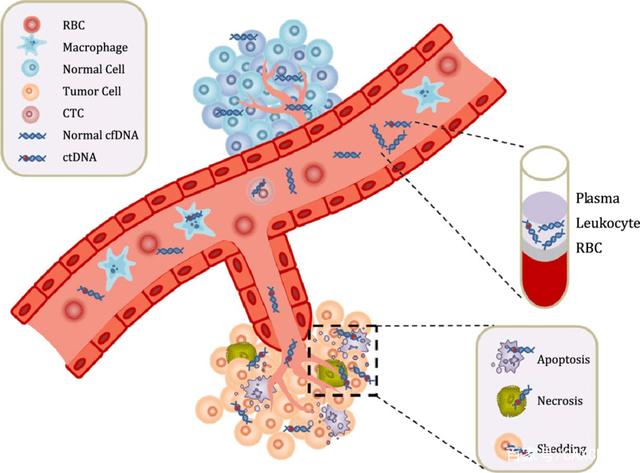
The cfDNA released by circulating tumor cells in plasma or in situ tumor cells into circulation in tumor patients is also called ctDNA. The research directions of ctDNA in tumor diagnosis mainly include: detection of ctDNA mutation, tumor specific microsatellite changes, epigenetic changes, ctDNA integrity detection and virus DNA detection. CtDNA mutation detection can not only obtain the gene mutation results corresponding to tumor tissues, but also more sensitively and specifically detect typical characteristic mutations such as EGFR gene mutation of lung cancer, KRAS mutation of colorectal cancer and BRAF mutation of melanoma.
Studies have shown that the sensitivity of EGFR gene mutation in ctDNA of lung cancer patients can be as high as 80%, which is conducive to monitoring drug resistance genes. The sensitivity and specificity of KRAS gene mutation in plasma ctDNA of patients with metastatic colorectal cancer were as high as 87.2% and 99.2%.
The integrity detection of tumor ctDNA is also the focus of current research. The main principle of integrity detection is that long and complete DNA fragments will be released during tumor necrosis, while cfDNA released by normal apoptotic cells is shorter. Detection of the integrity of ctDNA can be used to predict the development and micrometastasis of early cancer. In addition, studying the loss of gene heterozygosity in ctDNA and the methylation level of ctDNA can also provide help for tumor specific diagnosis and disease risk prediction. The methylation detection of ctDNA is also helpful to the early diagnosis of tumor.
Noninvasive prenatal testing:
CfDNA detection is also widely used in noninvasive prenatal testing (NIPT). The method for detecting cfDNA by NIPT is mainly developed based on next-generation sequencing technology (NGS), including random high-throughput parallel sequencing, target or chromosome specific sequencing and single nucleotide polymorphism sequencing. Fetal cfDNA in maternal plasma accounts for more than 10% of the total cfDNA in maternal plasma. Detecting these fetal cfDNA can determine the fetal RhD blood group, gender, chromosomal aneuploidy disease, single gene genetic disease and other related information. In addition, this method of detecting maternal plasma fetal cfDNA is less traumatic than invasive methods such as amniocentesis or villus biopsy, which greatly reduces the risk of complications such as intrauterine infection and abortion.
At present, it is mainly used to screen and judge common aneuploid chromosome diseases, including trisomy 21 syndrome, trisomy 18 syndrome and trisomy 13 syndrome. In addition, it has also been reported in sex chromosome aneuploidy diseases, chromosome microdeletions, copy number abnormalities and some single gene genetic diseases. Studies found that noninvasive prenatal screening cfDNA has high sensitivity (greater than 99%) and specificity, while the false positive rate is relatively low (less than 0.1%).
The international society for prenatal diagnosis (ISPD), the American College of Obstetricians and Gynaecologists (ACOG) and the American College of medicalgenetics and genomics (ACMG) all recommend pregnant women to carry out NIPT testing which helps the early diagnosis of chromosomal aneuploidy diseases (such as trisomy 21 syndrome) and monogenic genetic diseases during pregnancy.
Cardiovascular disease testing:
CfDNA mainly comes from apoptotic or necrotic cells. In this case, the content levelin plasma will increase due to the release of cfDNA into the blood. Coronary heart disease often leads to cardiomyocyte necrosis and apoptosis caused by long-term severe myocardial ischemia. Especially when myocardial infarction (MI) occurs, in addition to the increase of cfDNA caused by cardiomyocyte necrosis and apoptosis caused by myocardial ischemia, the delay and obstacle of cfDNA clearance are also related to the pathogenesis of the disease. The increase of plasma cfDNA in patients with coronary heart disease can be used to supplement the diagnostic results of cardiac troponin I (cTnI) and creatinekinase MB (CK-MB). Studies have shown that the plasma cfDNA concentration inpatients with MI is more than 10 times higher than that in healthy controls.

The concentration of cfDNA can increase in the early stage of myocardial injury (much earlier than troponin), and the peak time is later than CK-MB, which can be used to detect and judge myocardial injury in the early stage. In addition, cfDNA level is also a potential indicator to monitor disease progression and patientprognosis. The follow-up results showed that the level of cfDNA in plasma of MI patients with adverse cardiac results increased by more than 3.5 times.
Magen Biotechnology has developed wide range of Nucleic Acid Isolation Kits for Liquid Biopsy/NIPT/FFPE/Blood/Tissue sample preparation.
| Sample | Product Name |
Cat. No. |
Description |
| Blood DNA Kit | HiPure Univesal DNA Kit (column) | IVD3018 | Isolation DNA from tissue, cells, blood, saliva, swabs, blood spots, semen and other clinical samples |
| MagPure Universal DNA Kit (magnetic) | IVD3102 | ||
| Circulating DNA Kit | HiPure Circulating DNA Kit (vacuum) | IVD3182/D3182C | Isolation circulating DNA from 1-5ml plasma, serum, body fluids using vacuum protocol |
| MagPure Circulating DNA Mini Kit (magnetic) | IVD5432 | Isolation circulating DNA from 0.2-0.6ml plasma, serum, body fluids | |
| MagPure Circulating DNA Maxi Kit (magnetic) | IVD5435 | Isolation circulating DNA from 1-6ml plasma, serum, body fluids | |
| FFPE DNA Kit | HiPure FFPE DNA Kit (column) | IVD3126 | Isolation high pure total DNA from FFPE samples |
| MagPure FFPE DNA Kit (magnetic) | IVD6323B | ||
|
Raw Material |
MagPure Particles G | C1411 | Magnetic Beads for cell free DNA/gDNA/FFPE DNA isolation |
| MagBind Particles | C1413 | Magnetic Beads for micro/trace amount DNA/RNA isolation | |
| Machine | MagRotex 24 LV Nucleic acid Extractor | MagRotex 24 | Isolate cfDNA from 2-4ml plasma/serum, 24 channels, used for tumor diagnostic, liquid biopsy |
| MagRotex 24 Plus LV Nucleic acid Extractor | MagRotex 24 Plus | Isolate cfDNA from 2-9ml plasma/serum, 24 channels, used for tumor diagnostic, liquid biopsy | |
|
MagMix 96 Nucleic acid Extractor |
MagMix 96 | Isolate cfDNA from 1-500μl plasma/serum, 96 channels, used for tumor diagnostic, liquid biopsy |
Magen Kits public articles (click to visit):
- 1. A Noninvasive Multianalytical Approach for Lung Cancer Diagnosis of Patients with Pulmonary Nodules
- 2. Detection of ctDNA in the plasma of patients with papillary thyroid carcinoma
- 3. An optimized rapid bisulfite conversion method with high recovery of cell-free DNA
- 4. Detection of fetal trisomy and single gene disease by massively parallel sequencing of extracellular vesicle DNA in maternal plasma: a proof-of-concept validation
- 5. Cell-free DNA induced apoptosis of granulosa cells by oxidative stress